Millions of Americans each year face vision loss connected to diabetes. In truth, inning accordance with current information from the U.S. Centers for Disease Control and Avoidance (CDC), nearly 26 million Americans– roughly 8.3 percent of the United States population– have diabetes, and more than 28 percent of diabetics age 40 or older in the United States have diabetic retinopathy (DR) and associated diabetic eye disease.
To make matters worse, a substantial number of cases of diabetes and diabetic eye disease go undetected or unattended since people cannot have routine detailed eye examinations as advised by their eye doctor or ophthalmologist.
Most laser and non-laser treatments for diabetic eye disease depend upon the seriousness of the eye modifications and type of vision problems you have.
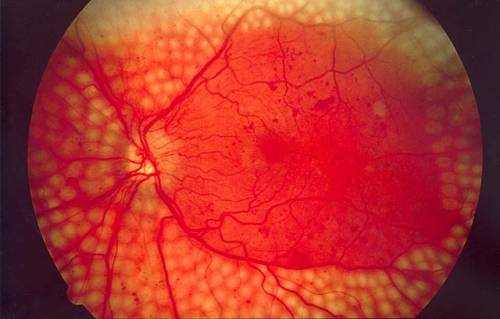
Diabetic retinopathy is diabetes-related damage to the light-sensitive retina in the back of the eye. As diabetes advances, chronic high blood sugar levels cause changes that harm the small capillary in the retina, makings them leak fluid or hemorrhage (bleed). Eventually, this results in vision issues that can not be fixed with glasses or contact lenses.
The look of diabetic retinopathy is related to the proliferation of a protein called vascular endothelial growth aspect (VEGF) in the retina. VEGF stimulates the production of new members vessels in the retina to bring more oxygen to the tissue since retinal blood flow is inadequate due to diabetes.
Unfortunately, these tiny new members vessels that form in the retina in reaction to VEGF are delicate and boost in number, causing additional fluid leakage, bleeding and scarring in the retina and progressive vision loss.
Blood vessel leakage from diabetic retinopathy can cause fluid to build up in the macula, which is the most sensitive part of the retina that is responsible for central vision and color vision.
This condition– called diabetic macular edema (DME)– is the primary reason for vision loss associated with diabetic retinopathy and is the leading reason for brand-new cases of blindness in adults ages 20 to 74 in the United States, inning accordance with CDC.
Lasers For Diabetic Retinopathy Treatment
Laser treatment of diabetic eye disease generally targets the broken eye tissue. Some lasers treat leaking capillary directly by “spot welding” and sealing the area of leak (photocoagulation). Other lasers get rid of unusual blood vessels that form from neovascularization.
Lasers also may be used to deliberately destroy tissue in the periphery of the retina that is not needed for functional vision. This is done to enhance blood supply to the more essential central portion of the retina to preserve sight.
The peripheral retina is believed to be involved in development of VEGF accountable for unusual blood vessel development. When cells in the peripheral retina are destroyed through panretinal photocoagulation (see listed below), the quantity of VEGF is decreased, together with the possible to produce abnormal retinal blood vessels.
After laser treatment of the peripheral retina, some blood flow bypasses this area and rather provides extra nourishment to the central portion of the retina. The resulting increase of nutrients and oxygen helps preserve the health of cells in the macula that are essential for comprehensive vision and color understanding. Nevertheless, some peripheral vision might be lost due to this treatment.
The two types of laser treatments frequently used to treat considerable diabetic eye disease are:
- Focal or grid laser photocoagulation. This type of laser energy is aimed directly at the affected area or used in a contained, grid-like pattern to ruin broken eye tissue and clear away scars that add to blind spots and vision loss. This approach of laser treatment usually targets particular, private blood vessels.
- Scatter (panretinal) laser photocoagulation. With this technique, about 1,200 to 1,800 tiny spots of laser energy are applied to the periphery of the retina, leaving the main area untouched.
Treatment of medically substantial DME also involves using fluorescein angiography to supply images of the eye’s interior. These images properly direct application of laser energy, which helps “dry up” the localized swelling in the macula. A fluorescein angiogram also can identify the area of blood vessel leak triggered by proliferative diabetic retinopathy.
While laser treatment for diabetic retinopathy usually does not enhance vision, the therapy is designed to prevent further vision loss. Even people with 20/20 vision who satisfy treatment guidelines need to be thought about for laser therapy to prevent ultimate vision loss related to diabetes.
What To Expect Before, During and After Laser Treatment
Laser treatment generally requires no overnight health center stay, so you will be treated on an outpatient basis in a clinic or in the eye doctor’s workplace.
Ensure you have somebody drive you to and from the office or center on the day you have the procedure. Likewise, you’ll have to wear sunglasses afterward because your eyes will be momentarily dilated and light delicate.
Prior to the procedure, you will receive a topical anesthetic or potentially an injection surrounding to the eye to numb it and prevent it from moving during the laser treatment.
Your optometrist will make these types of modifications to the laser beam prior to it is intended into the eye:
- The quantity of energy used
- The size of the “spot” or end of the beam that is directed into the eye
- The pattern used by the laser beam onto the targeted area
A laser treatment usually lasts a minimum of several minutes, but more time may be needed depending on the degree of your eye condition.
During laser treatment, you may experience some pain, but you should feel no pain. Right after a treatment, you must have the ability to resume normal activities. You might have some pain and blurry vision for a day or two after each laser treatment.
The variety of treatments you require will depend upon your eye condition and degree of damage. People with scientifically substantial diabetic macular edema might require three to four various laser sessions at two- to four-month periods to stop the macular swelling.
Though the specific mechanism by which laser photocoagulation lowers diabetic macular edema is not completely comprehended, a landmark study called the Early Treatment Diabetic Retinopathy Study (ETDRS) showed that focal (direct/grid) photocoagulation lowers moderate vision loss brought on by DME by 50 percent or more.
In December 2011, Iridex Corporation announced the outcomes of a 10-year study of the company’s MicroPulse laser therapy for treating DME. The study data showed the brand-new micropulse technology was at least as efficient as traditional laser photocoagulation in the treatment of macular edema, with less risk of thermal damage and scarring to the surrounding retinal tissue.
If you have proliferative diabetic retinopathy (PDR)– meaning that leakage of fluid has actually started in the retina– the laser treatment should draw from 30 to 45 minutes per session, and you might require up to 3 or four sessions.
Your possibility of maintaining your remaining vision when you have PDR enhances if you receive scatter laser photocoagulation as soon as possible following diagnosis.
Early treatment of PDR particularly is effective when macular edema also exists.
Non-Laser Treatment Of Diabetic Macular Edema
Injection of corticosteroids or other medications into the eye– either straight or in the form of an injectable implant– is often advised over laser treatments for the treatment of diabetic macular edema. Or in many cases, a combination of drug injections and laser treatment might be recommended.
As diabetic retinopathy worsens, in addition to VEGF, other small “signal” proteins (cytokines) are released by cells, causing extra inflammation in the retina that can cause or get worse DME. Corticosteroids have been shown to have a beneficial result by decreasing the quantity of VEGF and other inflammatory cytokines produced by cells (a procedure called “downregulation”), which can result in a decrease of diabetes-related macular edema.
Though the following medications decrease levels of numerous proteins associated with swelling, they are typically categorized as “anti-VEGF” medications.
Anti-VEGF drugs or drug-releasing implants that are FDA-approved for injection into the eye for treatment of DME in the United States consist of:
- Iluvien (Alimera Science).
- Ozurdex (Allergan).
- Lucentis (Genentech).
- Eylea (Regeneron).
Iluvien is a tiny implant that provides a continual, sluggish release of a corticosteroid (fluocinolone acetonide) to treat diabetic macular edema. It is prescribed for patients who formerly have actually been treated with corticosteroids and did not have a scientifically substantial increase in intraocular pressure (a prospective side effect of corticosteroid use).
Iluvien received FDA approval in September 2014, based on medical trial information that revealed that patients getting the implant showed a statistically significant enhancement in visual acuity within 3 weeks of the procedure, compared with a control group; and at 24 months after the procedure, 28.7 percent of patients revealed an enhancement in visual acuity of 15 letters or more on a standardized eye compared to standard (prior to going through the procedure).
Inning accordance with Alimera Sciences, a significant benefit of Iluvien over other treatments for DME is the durability of its result: Iluvien is created to provide a sustained release of corticosteroid medication for 36 months (3 years), compared with other treatments that last only a month or two.
Ozurdex, another FDA-approved implant for DME treatment, releases a sustained dosage of dexamethasone (a corticosteroid) to the retina. In September 2014, Ozurdex received approval for all patients with diabetic macular edema. Previously, the device was approved to treat DME just among adult patients who likewise previously had actually or were scheduled to have cataract surgery with intraocular lens (IOL) implantation.
The Ozurdex implant likewise is FDA-approved for treatment of posterior uveitis and for macular edema following branch retinal vein occlusion (BRVO) or central retinal vein occlusion (CRVO)– two types of eye strokes.
Lucentis (ranibizumab), marketed by Genentech, got FDA approval for the treatment of diabetic macular edema in 2012 and was approved for the treatment of diabetic retinopathy (with or without DME) in April 2017.
Approval of Lucentis to treat DME was based on scientific trials that showed up to 42.5 percent of patients who were given month-to-month eye injections of the drug got a minimum of 15 letters in best fixed visual skill (BCVA) on a basic eye chart two years after initiation of the treatment, compared to 15.2 percent of patients in a control group.
Another study discovered that Lucentis injections and Lucentis injections combined with laser photocoagulation both were significantly more effective than laser treatment alone for the treatment of DME.
Approval of Lucentis for the treatment of diabetic retinopathy with or without DME was based on outcomes of several medical studies that showed the drug showed a considerable improvement of patients’ diabetic retinopathy, according to Genentech.
Eylea (aflibercept) is another anti-VEGF drug that is FDA-approved for the treatment of DME. It is likewise approved for treatment of innovative age-related macular degeneration (AMD) and macular edema following retinal vein occlusion.
The FDA’s approval of Eylea for DME treatment was based on one-year data from two studies of 862 patients, which assessed eye injections of 2 mg of Eylea administered either monthly or every two months (after 5 initial regular monthly injections). Results were compared to patients who were treated solely with laser photocoagulation (once at the beginning of the study and after that as required).
The two Eylea treatment procedures produced similar outcomes, which were considerably much better than those produced by laser treatment. Patients in both Eylea treatment groups gained, usually, the ability to check out roughly two extra lines on an eye chart, compared to almost no change in visual acuity in the control group.
The recommended dose for Eylea is 2 mg administered by injection into the eye every two months (following 5 initial regular monthly injections).
Retisert (Bausch + Lomb) is another intraocular implant that provides long-lasting, continual release of a corticosteroid (fluocinolone acetonide). Presently, Retisert is FDA-approved for the treatment of posterior uveitis, but some eye surgeons also use the device “off label” for the treatment of DME.
Retisert is developed to provide corticosteroid therapy inside the eye for as much as 2.5 years, inning accordance with Bausch + Lomb. The device is implanted into the eye through a surgical incision in the sclera.
Risks connected with intraocular steroid treatment for DME include steroid-induced cataracts and glaucoma. Vision loss from cataracts usually can be brought back with cataract surgery. To reduce the risk of glaucoma, your eye doctor may recommend preventive use of glaucoma eye drops or perhaps glaucoma surgery.
Vitrectomy And Other Surgery Treatments For Diabetic Eye Disease
In some people who have proliferative diabetic retinopathy, bleeding into the vitreous (vitreous hemorrhage) makes laser photocoagulation treatment impossible because the blood obscures the surgeon’s view of the retina.
If the vitreous hemorrhage cannot clear within a few weeks or months, a vitrectomy surgery may be carried out to mechanically eliminate the hemorrhage– after which, laser photocoagulation can be used. The laser procedure is performed either at the time of the vitrectomy or soon thereafter.
Retinal bleeding and vitreous hemorrhage likewise can cause bands of scar tissue to form. These bands of scar tissue can shrink and– if attached to the retina– can cause the retina to pull away from its base to develop traction.
This traction may lead to retinal tears or possible retinal detachments.
If you experience a tractional detached retina as part of PDR and diminishing scar tissue that moves the retina, you typically will be scheduled promptly for a procedure to reattach the retina.
ETDRS guidelines show that type 2 diabetics in particular can lower their opportunity of severe vision loss and the need for vitrectomy surgery by about 50 percent when proliferative diabetic retinopathy is dealt with prior to it reaches a high-risk stage.
Steroid Eye Drops For Diabetic Macular Edema
Some people with diabetic macular edema may experience reduced symptoms and enhanced vision after treatment with corticosteroid medication delivered to the eye through eye drops instead of an intraocular implant.
In a study published in Acta Ophthalmologica in November 2012, researchers found that patients with scattered DME who used Durezol emulsion eye drops (Alcon) four times a day for one month had actually decreased retinal swelling and a considerable enhancement in visual skill, compared with comparable DME patients who did not use the eye drops.
Durezol is a corticosteroid eye drop used primarily for the treatment of swelling and pain related to eye surgery.
The research study authors concluded that use of Durezol eye drops is a beneficial and effective treatment for diffuse DME without surgical intervention and the associated risk of possibly severe side effects.