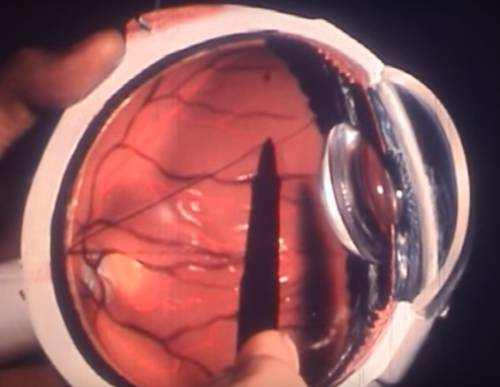
All glaucoma surgery procedures (whether laser or non-laser) are developed to accomplish one of two fundamental outcomes: reduce the production of intraocular fluid (liquid humor) or increase the outflow (drain) of this same fluid. Periodically, a procedure will accomplish both.
Presently the objective of glaucoma surgery and other glaucoma treatment is to reduce or support intraocular pressure (IOP). When this objective is achieved, damage to ocular structures– particularly the optic nerve– might be prevented.
No matter the treatment, early medical diagnosis is the best way to avoid vision loss from glaucoma. See your eye care professional consistently for a total eye evaluation, consisting of a check of your IOP. People at high risk for glaucoma due to elevated intraocular pressure, family history, ethnic background, age or optic nerve look might require more regular visits to the eye doctor.
When Is Glaucoma Surgery Needed?
Depending on the type of glaucoma you have, different treatment options might be thought about.
Non-surgical choices include using topical eye medications (glaucoma eye drops) or oral medications (pills).
Most cases of glaucoma can be controlled with one or more drugs. However some people might require surgery to decrease their IOP further to a safe level by improving the outflow or drainage of fluids. Occasionally, surgery can remove the requirement for glaucoma eye drops. Nevertheless, you may need to continue with eye drops after having glaucoma surgery.
Types of Glaucoma Surgery
Some current research studies suggest that a laser procedure called selective laser trabeculoplasty (SLT) might be similarly as reliable as glaucoma eye drops for decreasing internal eye pressure. This laser surgery might be thought about a primary treatment, especially for people who find it hard to adhere to the rigorous, regular schedule required for administering eye drops.
Another procedure called a trabeculectomy creates a synthetic drainage area. This technique is used in cases of innovative glaucoma where optic nerve damage has taken place and the IOP continues to soar. A third typical option is a shunt, a device that a surgeon implants in your eye to enhance fluid drain.
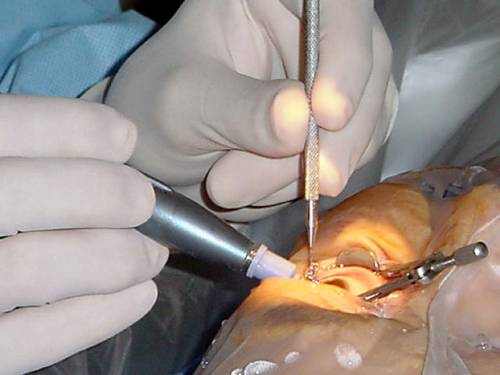
Laser Trabeculoplasty
To increase outflow of internal eye fluid, an eye doctor performs laser trabeculoplasty with a laser that produces tiny holes in the filtration angle of the eye, where the cornea and iris meet.
A more recent procedure, selective laser trabeculoplasty, creates minimal heat damage to surrounding tissue, which usually indicates the procedure can be repeated safely.
Laser trabeculoplasties are typically performed as an accessory to a patient’s ongoing eye drop therapy.
Studies are now examining whether SLT could be used consistently as a first-line therapy for treatment of open-angle glaucoma and other types of glaucoma, even before eye drops are used.
Trabeculectomy, Trabeculotomy and Goniotomy
Your optometrist might suggest that a surgical incision be made into the eye’s drain system to develop brand-new channels for more normal flow of fluid. To accomplish this objective, a trabeculectomy includes partial removal of the eye’s drainage system.
Trabeculectomy is the most common surgical non-laser procedure performed for glaucoma when the IOP is no longer controlled by eye drops, pills or laser trabeculoplasties.
A trabeculectomy produces a “controlled” leak of fluid (liquid humor) from the eye, which percolates under the conjunctiva. A little conjunctival “bleb” (bubble) appears at the junction of the cornea and the sclera (limbus) where this surgically produced valve is made.
A trabeculotomy is the exact same as a trabeculectomy, other than that cuts are made without elimination of tissue.
A goniotomy normally is used for infants and small children, when an unique lens is required for seeing the inner eye structures to produce openings in the trabecular meshwork to allow drain of fluids.
Iridotomy and Iridectomy
In an iridotomy, a laser is used to create a hole in the iris to enhance the drain passages blocked by a part of the iris.
An iridectomy involves surgically removing a small piece of the iris that will permit a better flow of fluid in eyes with narrow-angle glaucoma.
Shunts and Implants for Glaucoma
Glaucoma shunts and stents are small devices that are surgically placed into the eye during a trabeculectomy to increase outflow of intraocular fluid and reduce high eye pressure.
The devices, typically made from products such as silicone, polypropylene or biocompatible metals, create an alternative passageway for the aqueous to get away from the eye, bypassing the eye’s harmed or clogged filtration drain canals.
The term “glaucoma implants” sometimes is used to explain shunts and stents, however also describes small devices implanted in the eye that are designed to offer a sustained release of glaucoma medication to lower eye pressure.
Complications of these implants can consist of creating a pressure that is too low for the eye to function (hypotony). Implants also can be placed too near the front of the eye’s surface, triggering corneal disintegration. They also can cause disintegrations in the eye tissues where they have been positioned.
In spite of these risks, shunts and implants for glaucoma usually are safe and effective and can decrease or get rid of the requirement for daily glaucoma medications.
Shunts and implants that have gained FDA approval for glaucoma surgery in the U.S. or that presently are in medical trials consist of:
- Ex-Press Glaucoma Filtration Device. Offered by Alcon, this is a miniature, stainless-steel shunt for glaucoma surgery that has actually been FDA-approved given that 2002. About the size of a grain of rice, the Ex-Press shunt is implanted under a small flap developed in the sclera and allows the aqueous to bypass the harmed trabecular meshwork and exit the eye more easily to lower IOP.
- In a research study released in early 2012 that compared implantation of the Ex-Press Glaucoma Filtration Device with standard trabeculectomy surgery, both treatments provided comparable IOP control, but the Ex-Press group had a lower rate of complications and required less glaucoma medication after surgery.
DeepLight Glaucoma Treatment System. Established by SOLX, the DeepLight Glaucoma Treatment System combines making use of titanium sapphire laser energy to open the filtering angle of the eye and insertion of a hollow gold micro-shunt. The shunt creates a synthetic channel to allow fluid drainage and eliminate eye pressure. The laser and the shunt also can be used separately. - The SOLX laser system, which received FDA approval in September 2008, is similar to selective laser trabeculoplasty (SLT), because just pigmented cells are targeted, sparing adjacent tissue from prospective heat damage. The DeepLight Gold Micro-Shunt runs differently from other types of glaucoma implants, since drain is restricted to the eye’s interior with the idea of reducing surgical complications. The combined system has CE mark accreditation for use in Europe and presently is undergoing FDA clinical trials in the United States.
- iStent Trabecular Micro-Bypass. This shunt system from Glaukos Corp. is offered in Europe for the treatment of open-angle glaucoma. The device likewise is commercially readily available in the United States and Canada for use in combination with cataract surgery for the decrease of IOP in patients with moderate to moderate open-angle glaucoma. Made of surgical-grade titanium, the stent is positioned in an internal area of the eye known as Schlemm’s canal to re-establish a more normal circulation of fluids within the eye.
- Durasert. In June 2011, pSividia Corp. announced an early stage clinical trial of its Durasert glaucoma implant is underway in the United States. The bioerodible implant is inserted under the scleral conjunctiva and is designed to provide long-term sustained release of the glaucoma medicine latanoprost, minimizing or getting rid of the requirement for daily medicated eye drops to treat glaucoma.
- CyPass Micro-Stent. In July 2011, Transcend Medical announced it had secured additional financing for a big U.S. medical research study and continuous global trials of its CyPass Micro-Stent device. The CyPass device is developed to be placed in the eye during routine cataract surgery for cataract patients who likewise have open angle glaucoma. More info about the United States scientific research study of the device is offered on the COMPASS Clinical Study site.
- Hydrus Microstent. This small implant, being established by Ivantis, is roughly the size of an eyelash and is being checked in the U.S. for the treatment of primary open-angle glaucoma. The Hydrus procedure is less invasive than traditional glaucoma surgery and can be carried out during cataract surgery using the very same microsurgical cuts, inning accordance with the company.
At the 2012 yearly meeting of the American Academy of Ophthalmology, Thomas Samuelson, MD, reported first-year outcomes of a Phase 3 FDA trial of the device, which revealed that glaucoma patients going through the Hydrus procedure needed 69 percent less medication to manage their eye pressure after the surgery.
- Xen Gel Stent. This collagen-derived, gelatin implant is currently an investigational device in the United States. Its maker, AqueSys, states the pliability and softness of the device enable it to comply with the ocular tissue, possibly mimizing issues seen with artificial materials. Xen Gel Stent is authorized in Canada and the European Union for use with or without cataract surgery.
Nonpenetrating Glaucoma Surgery (NPGS)
Different ingenious surgical methods change the eye’s drainage channels, improving the circulation of fluids with only minimal penetration into the eye.
These surgical approaches involve superficial cuts that do not penetrate the eye as deeply as, for example, a trabeculectomy. Advocates state fewer complications are most likely to arise from these less invasive treatments.
A deep sclerectomy includes a minimally invasive incision into the white of the eye (sclera), a portion of which is gotten rid of to develop a drainage area for relief of eye pressure.
A brand-new surgical method known as viscocanalostomy develops an opening for insertion of a highly flexible, gel-like material referred to as viscoelastic, which helps offer adequate space for adequate drainage and eye pressure relief.
The Future of Glaucoma Treatment
Glaucoma professionals have differing viewpoints about the use of drug, laser and surgical intervention to control high IOP.
Some glaucoma experts, for example, state that long-term expenses of drug treatments including eye drops can be a financial burden that might be balanced out with using laser treatments.
Others argue that treatments such as eye drops are far less invasive, are normally reliable and have fewer risks of complications than laser or non-laser surgical techniques. Study results comparing long-lasting efficiency of various treatments also vary.